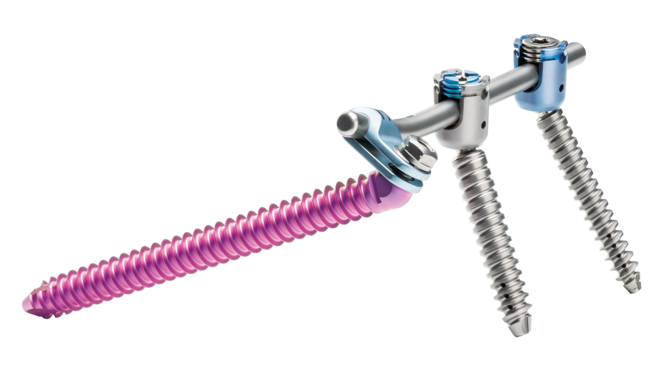
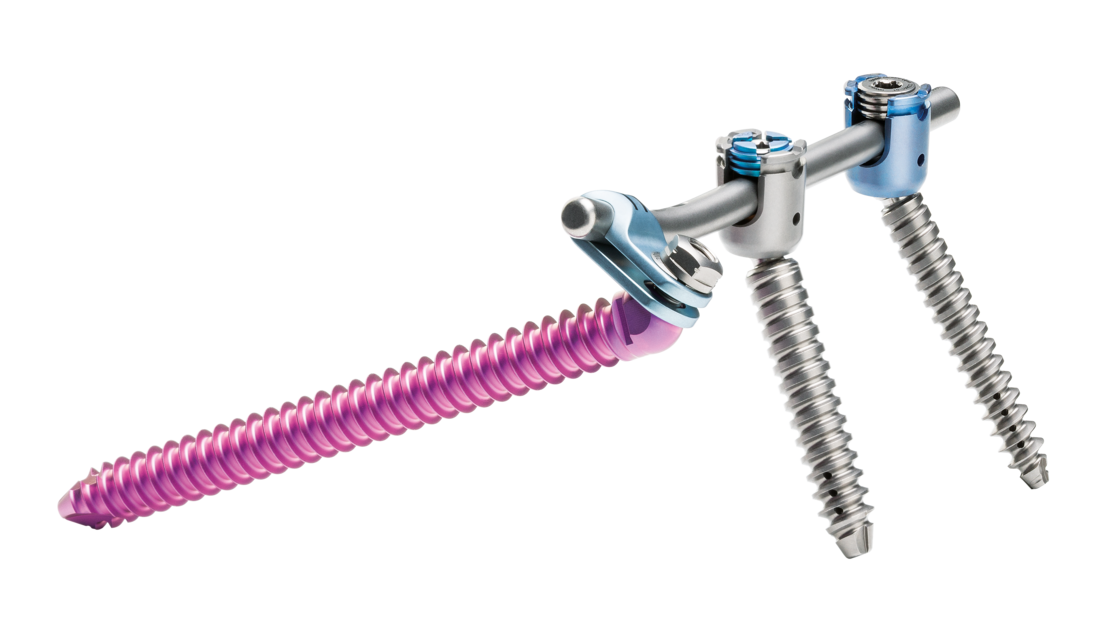
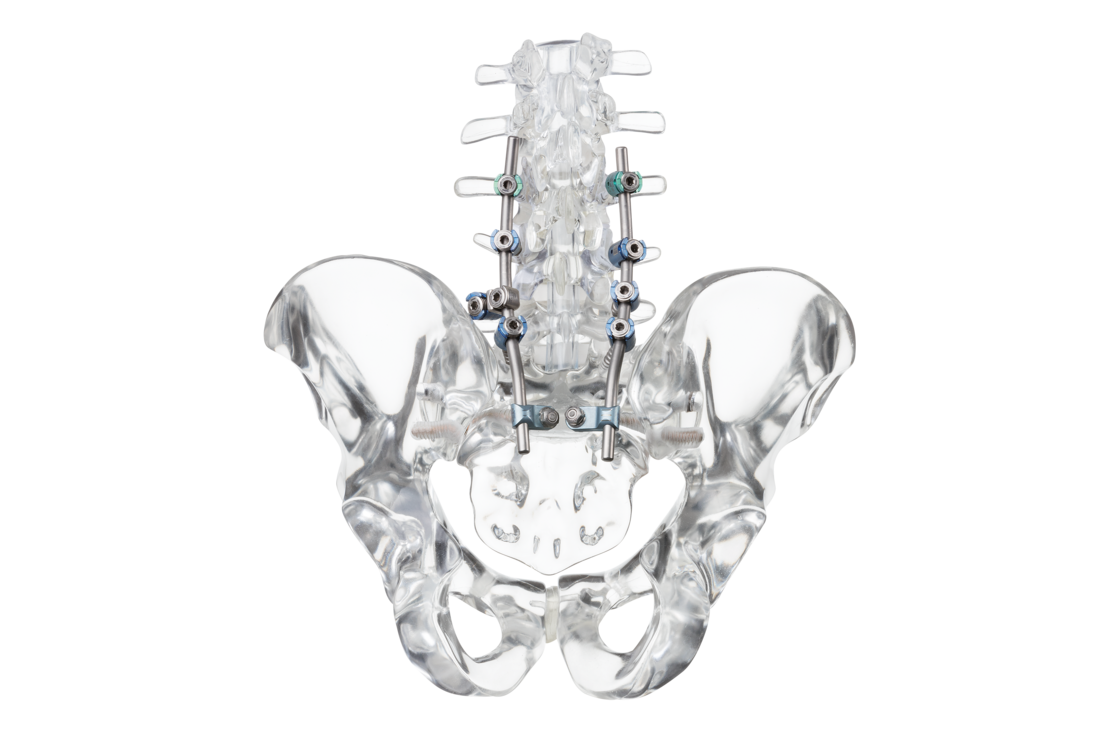
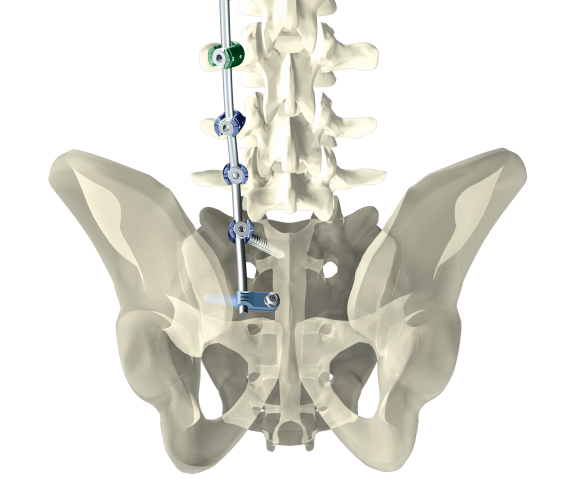
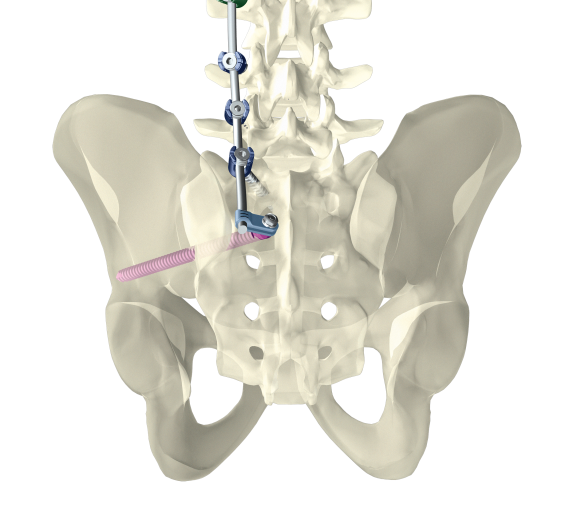
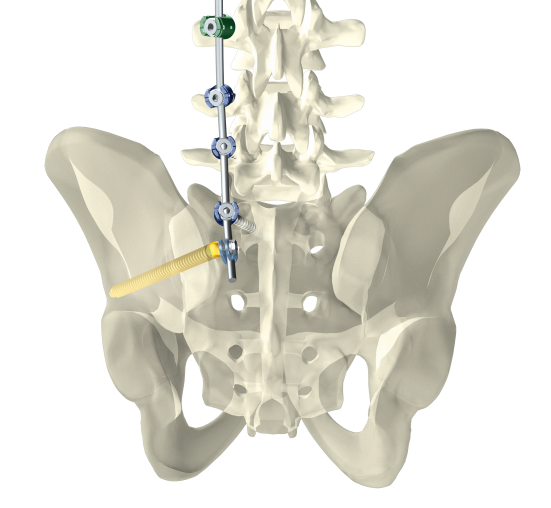
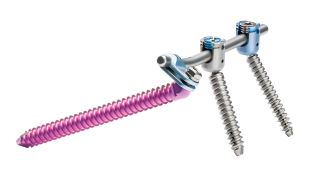
Dorsal stabilization of the sacrum and/or ilium
Looking for an iliosacral screw-rod system for dorsal stabilization of the sacrum and/or ilium to offer your patients a safe procedure tailored to their needs?
uBase is an implant system for dorsal surgical stabilization and fixation of the adult human sacrum and/or ilium in combination with the ulrich medical internal lumbosacral fixator.