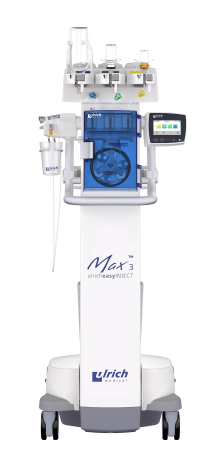
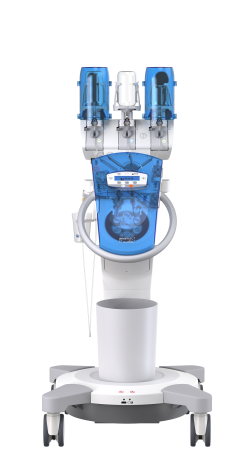
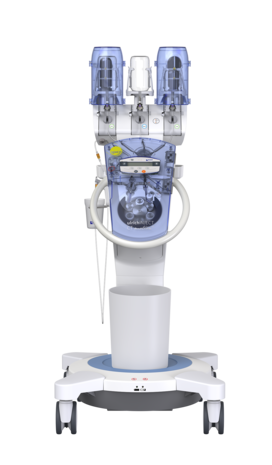
GLP-compliant study: “Microbiological Contamination Control Study of the ulricheasyINJECT Max 3 System”, report 12046467, dated: February 28, 2018, author: UL International GmbH (Grenzenstr. 13, 88416 Ochsenhausen, Germany); study is unpublished, but available on request with nondisclosure agreement.
GLP-compliant study: “Virological evaluation of Retrograde Contamination Potential at the Pump Tubing Set (XD 8003) and the Patient Tubing Set (XD 2035) of the CT motion (XD 8000)”, Report 10874251 2.1, dated: November 12, 2015, author: UL International GmbH (Grenzenstr. 13, 88416 Ochsenhausen, Germany); study is unpublished, but available on request with nondisclosure agreement.
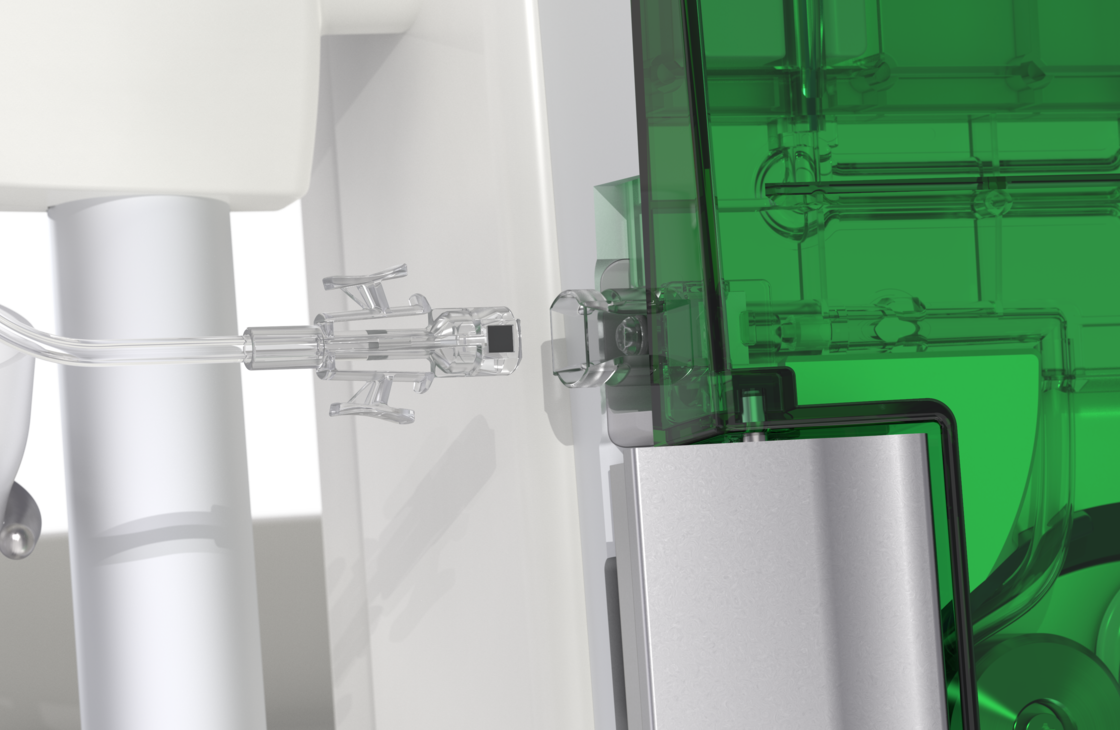
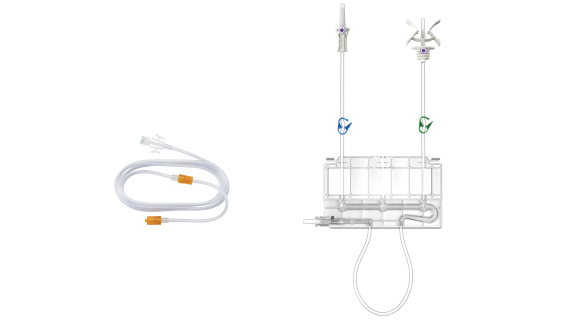
The results of the non-clinical testing mentioned are applicable to the ulricheasyINJECT systems Max 3 and Max 2M as the physical barrier with respect to possible retrograde contamination is implemented in all of the systems by means of structurally identical two check valves.