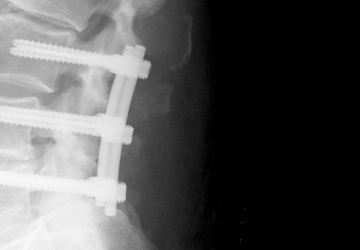
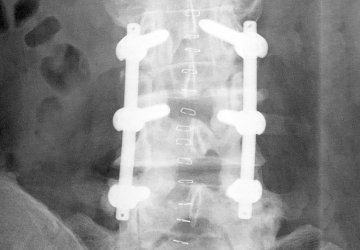
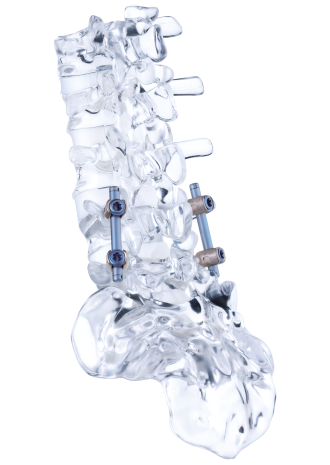
Dynamic screw-rod system
Comparative study: dynamic spine stabilization as an alternative to fusion
Introduction: Similar functional results with dynamic pedicle-based stabilization and fusion – this is the result of a study recently published in the Journal of Neurosurgery [1]. As a less complex procedure, the authors classify dynamic stabilization as a promising alternative to fusion.
Fusion with a rigid system is seen as the standard treatment for degenerative symptomatic instability of the lumbar spine. The aim of this multicenter, prospective, double-blind, randomized controlled study was to determine whether dynamic stabilization involves improved function while reducing surgical complexity and, therefore, surgery time and perioperative complications compared with lumbar fusion. To this end, data from 269 patients was analyzed, 137 of whom were treated with a rigid system and 132 with cosmicMIA.
Clinical endpoints and surgical data
After 24 months, there were no significant differences in the primary endpoint (Oswestry Disability Index, ODI) between the two stabilization methods. The study also showed that the two systems did not differ regarding procedure- and product-related adverse events or the number of revision surgeries after 24 months. Since dynamic stabilization with cosmicMIA is less surgically complex than fusion surgery, it could be proven to have significantly shorter surgery times, averaging approximately 30 minutes and approximately 10 percent lower hospital costs. Furthermore, intraoperative blood loss was significantly lower with cosmicMIA than with the rigid system, averaging approximately 120 ml.
“We can confirm the study results from everyday clinical practice”, says Prof. Dr. Ehab Shiban, Acting Director of Neurosurgery at University Hospital Augsburg. “Dynamic stabilization provides the same result for patients as fusion, but with a less complex surgical intervention. In our experience, the significantly shorter surgical time as well as less blood loss should not be underestimated.”
Patient selection, study setup
Patients had to meet the following inclusion criteria: diagnosed mono- or bisegmental instability of the lumbar spine with or without canal stenosis with failed conservative treatment for at least three months, indicated for fusion/stabilization, minimum age: 18 years. Dynamic stabilization was performed with the pedicle-based cosmicMIA dynamic stabilization system without interbody or posterolateral stiffening with or without decompression. The rigid fusion-promoting systems are not specified in more detail, only the surgical techniques rigid pedicle-based fixation and interbody fusion with a cage using transforaminal lumbar interbody fusion or posterior lumbar interbody fusion technique, with or without additional posterolateral fusion and decompression of the spinal canal.
The study is available at https://thejns.org/spine/view/journals/j-neurosurg-spine/37/4/article-p515.xml.
Further information on cosmicMIA is available at https://www.ulrichmedical.com/en/spinal-surgery/products/rod-screw-systems/cosmicmia.
[1] Meyer, B., Thomé, C., Vajkoczy, P., Kehl, V., Dodel, R., Ringel, F., & for the DYNORFUSE Study Group. (2022). Lumbar dynamic pedicle-based stabilization versus fusion in degenerative disease: a multicenter, double-blind, prospective, randomized controlled trial, Journal of Neurosurgery: Spine (published online ahead of print 2022). https://doi.org/10.3171/2022.2.SPINE21525 (accessed on November 28th, 2022)
Conflict of interest
This study was supported by a grant from ulrich GmbH & Co. KG. As described in the publication, ulrich medical was not involved in study planning, data collection and analysis, or interpretation of the data. ulrich medical also had no influence on writing and submitting the publication.